Estimated read time: 3-4 minutes
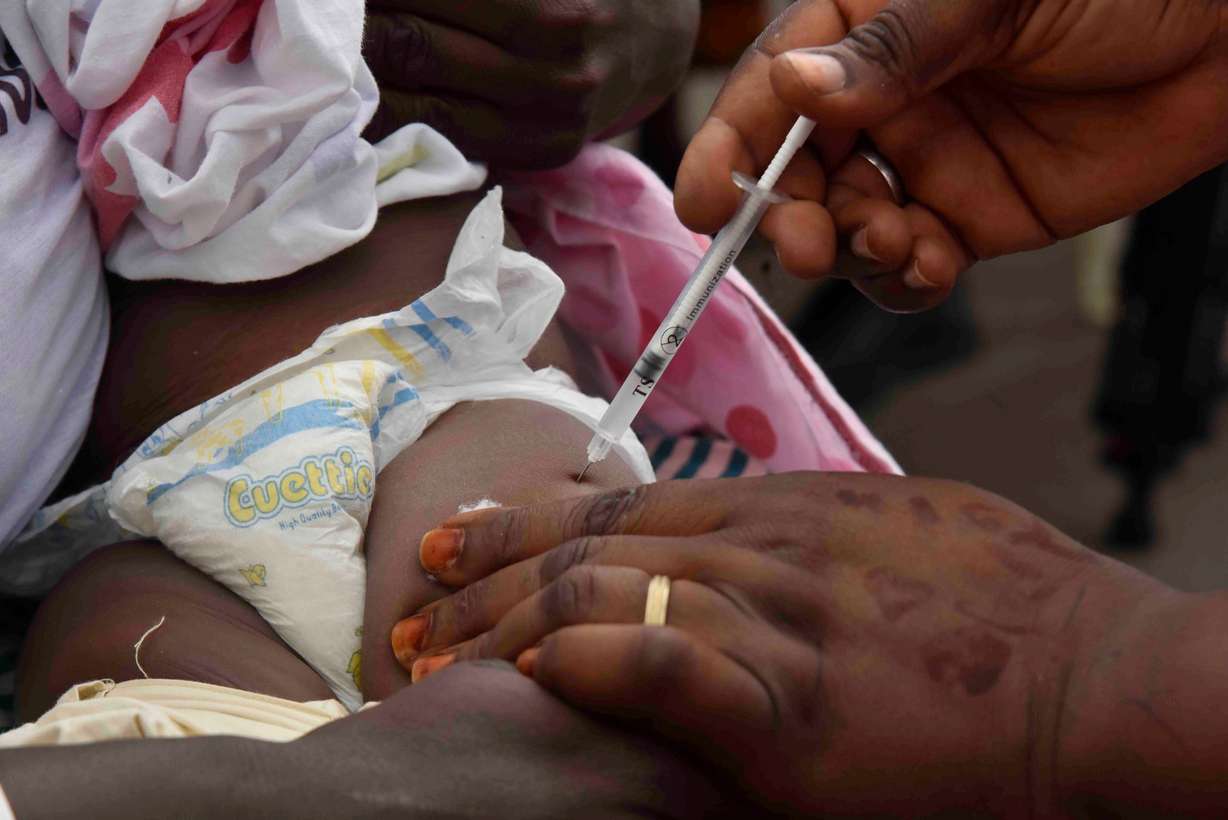
SALT LAKE CITY — Nearly half a million children younger than 5 die each year in Africa because of malaria, so great excitement accompanied the rollout July 15 of a second malaria vaccine — both released this year.
Côte d'Ivoire is the first adopter of the new vaccine, called R21, created by the University of Oxford and the Serum Institute of India. That government plans to make it part of its routine vaccine requirement. The first vaccine, which has been available for six months, was created by GSK and is part of a routine program in Cameroon, according to Reuters.
"Some 15 African countries plan to introduce one of the two malaria vaccines this year with support from the Gavi global vaccine alliance," per the article.
In an interview with Gavi, Côte d'Ivoire's director of immunization, Dr. Yao Kossia, said the malaria situation is dire for young children in that country, where 600 per 1,000 children under 5 got malaria in 2021. And at least a thousand die from it each year — roughly three a day. Pregnant women are also at high risk.
Sir Adrian Hill, a professor of vaccinology at University of Oxford, told the BBC he hopes the vaccine developed there leads to "really serious" efforts to wipe out malaria.
While the vaccines are not expected to replace routine steps families take to protect against malaria, like using mosquito nets that have been sprayed with insecticide and environmental efforts to keep mosquito populations down, the vaccines are seen as a game-changing and vital tool — especially since Kossia said the other measures are beginning to fail as mosquitoes carrying malaria become resistant to the insecticides.
Reuters said the new vaccine has also been approved by Burkina Faso, the Central African Republic, Ghana and Nigeria.
Still, there's a problem: It will likely be years before the supply of the two vaccines is ramped up adequately to meet all of the need. The Serum Institute of India, which makes the vaccine, said the rollout includes 25 million doses, which will quadruple by the end of the year. And the cost is less than $4 a dose to keep it affordable.
Hill said the low cost makes it "realistic to roll this out in many tens of millions of doses from now on. ... Instead of this being $9 or $10 (U.S.) dollars, as with the original vaccine per dose, this is just under $4, and that makes a real difference in low-income countries," he added.
What is malaria?
The Centers for Disease Control and Prevention calls malaria a serious disease that is caused by a parasite that infects certain mosquitoes. Most infections come from mosquito bites.
If it's not treated quickly, the person who is infected could die. Prompt treatment staves off serious illness and death.
While it's not usually found in the U.S., there are about 2,000 cases here a year, typically from travelers who return from trips where malaria is rampant. Occasionally, local spread happens, though malaria does not spread like the flu and is not sexually transmitted.
Last year, locally acquired malaria was found in several states, as Deseret News reported. It's locally acquired if the person who has it has not traveled to places where malaria is being transmitted.
Symptoms are miserable and can include fever, flu-like illness, chills, headaches and muscle aches, and exhaustion, as well as digestive ills like vomiting and diarrhea.
In severe cases, the CDC says symptoms include kidney failure, seizures, confusion and coma.
A large clinical trial reported earlier said the vaccine prevented about three-fourths of symptomatic malaria in young children in the year after they received a vaccine, as Reuters separately reported.
The Associated Press reported that protection is extended at least another year with a booster. The article said that "in 2021, WHO endorsed the first malaria vaccine, known as Mosquirix, made by GSK. But that vaccine requires four doses and protection fades within months. GSK also previously said it would only be able to make about 15 million doses."









